
Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.You can effortlessly find every single detail about the elements from this single Interactive Periodic table. Let me tell you how this Interactive Periodic Table will help you in your studies.ġ).
#Interesting facts about antimony free
Free Gift for you: Interactive Periodic Table

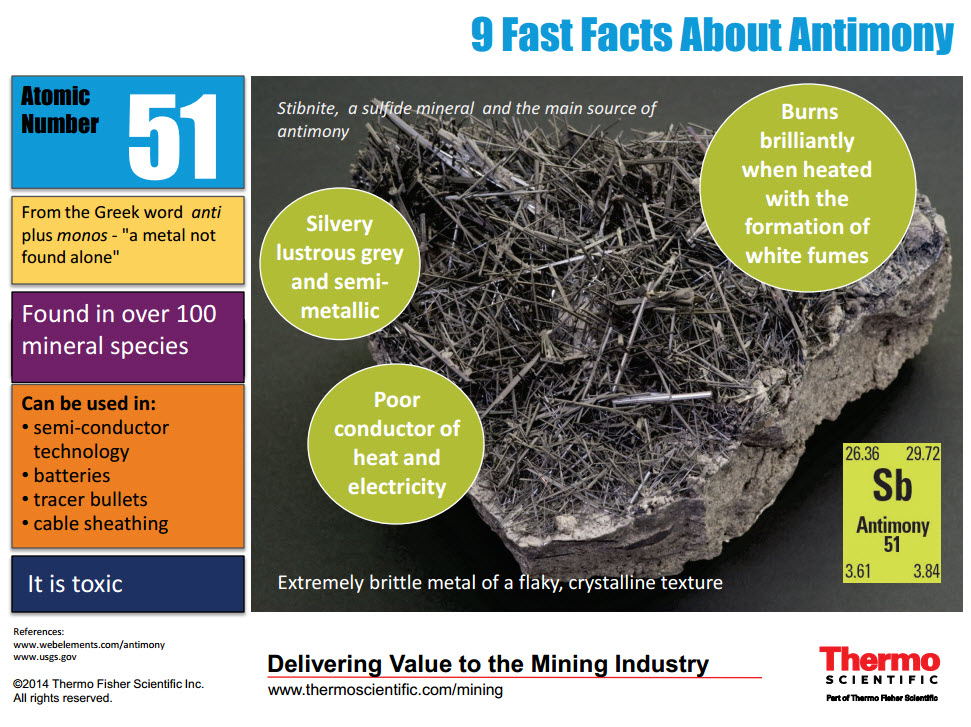
The name antimony was derived from the Greek words “anti” and “monos” which means a metal that is not found alone.Interesting facts about antimony element are mentioned below.

So the last electron of antimony enters the p-subshell or p-orbital. The simple answer: The elements will lie in the s, p, d or f block will completely depend upon the subshell in which the last electron will enter.įor example the electron configuration of antimony is 4d 10 5s 2 5p 3. How can you determine the blocks-wise position of elements? Rhombohedral Melting point of Antimony 903.7 K or 630.6 ☌ or 1167.1 ☏ Boiling point of Antimony 1908 K or 1635 ☌ or 2975 ☏ Density of Antimony 6.7 g/cm 3 Main isotope of Antimony 121Sb CAS number īefore knowing this reason, first of all I want to ask you a simple question. Protons in Antimony 51 Neutrons in Antimony 71 Electrons in Antimony 51 Symbol of Antimony Sb Atomic mass of Antimonyġ21.76 u Electrons arrangement in AntimonyĢ, 8, 18, 18, 5 Electronic configuration of Antimony 4d 10 5s 2 5p 3 Atomic radius of AntimonyĢ06 picometers (van der Waals radius) Valence electrons in Antimonyĥ 1st Ionization energy of Antimony 8.64 eV Electronegativity of AntimonyĢ.05 (Pauling scale) Crystal structure of Antimony Group: 15, Period: 5, Block: p Category of Antimony element Silvery gray metallic luster State of Antimony at STP Solid Position of Antimony in Periodic table Antimony Element (Sb) Information Appearance of Antimony So if you want to know anything about Antimony element, then this guide is for you. In fact, the table mentioned below is the perfect information box (Which gives you every single detail about the Antimony element in Periodic table.) This is a SUPER easy guide on Antimony element.


 0 kommentar(er)
0 kommentar(er)
